 |
LigASite database of binding sites |
|
PDB ID and HEADER, TITLE and
COMPND records of the PDB file. | | (click anywhere in this window to remove it) |
|
| 2cpl |
| ISOMERASE(PEPTIDYL-PROLYL CIS-TRANS) |
HEADER |
|
|
| SIMILARITIES AND DIFFERENCES BETWEEN HUMAN CYCLOPHILIN A AND OTHER BETA-BARREL STRUCTURES. STRUCTURAL REFINEMENT AT 1.63 ANGSTROMS RESOLUTION |
TITLE |
|
|
|
|
|
|
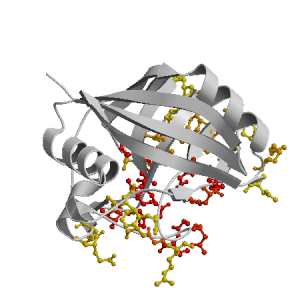
Figure highlighting the binding site residues. Figures were drawn with
Molscript (7) and rendered with
Raster3D (8). PISA coordinates
(3) are used when available
(all entries except NMR). | | (click anywhere in this window to remove it) |
|
 |
|
List of binding site residues detected in this protein. Column 1 gives the position, coloured on a yellow-to-red scale depending on the fraction of corresponding holo-structures where the residue is in contact with a ligand.
Column 2 gives the 3-letter amino acid code, coloured according to physico-chemical type. Chain ID's of residues are not mentioned in this page because all chains in the apo-structure refer to the same protein. | | (click anywhere in this window to remove it) |
|
| 54 | |
HIS |
| 55 | |
ARG |
| 57 | |
ILE |
| 58 | |
PRO |
| 59 | |
GLY |
| 60 | |
PHE |
| 61 | |
MET |
| 63 | |
GLN |
| 67 | |
PHE |
| 69 | |
ARG |
| 71 | |
ASN |
| 72 | |
GLY |
| 73 | |
THR |
| 74 | |
GLY |
| 75 | |
GLY |
| | 81 | |
GLU |
| 82 | |
LYS |
| 101 | |
ALA |
| 102 | |
ASN |
| 103 | |
ALA |
| 104 | |
GLY |
| 107 | |
THR |
| 109 | |
GLY |
| 110 | |
SER |
| 111 | |
GLN |
| 113 | |
PHE |
| 117 | |
ALA |
| 118 | |
LYS |
| 119 | |
THR |
| 121 | |
TRP |
| | 122 | |
LEU |
| 125 | |
LYS |
| 126 | |
HIS |
| 148 | |
ARG |
| 149 | |
ASN |
|
|
|
| PDB |
The Protein Data Bank |
| CSA |
Catalytic Site Atlas |
| PDBSum |
Overview of the macromolecular structure |
| CATH |
Protein Structure Classification |
| Scop |
Structural Classification of Proteins |
| Pfam |
Protein Families and Domains |
| UniProt |
Universal Protein Resource |
LIGPLOT (only on holo-pages) is hosted at the EBI. The LigPlot Jmol links point directly to the Jmol visualisation interface provided on the PDBSum page. Note that due to different software used, the atomic contacts of LigPlot and LigASite do not necessarily correspond. | | (click anywhere in this window to remove it) |
|
Links to external databases: |
|
Several files are provided for download: | • The XML file defining the residue-ligand contacts; this file contains data on the apo and all holo-structures. |
| • The XML Schema file defining the semantics of the XML file |
| • 3D coordinates of the structure used in constructing LigASite (PISA structure file whenever available, PDB file otherwise. |
| • 3D coordinates of the combined binding residues in the apo structure |
| • 3D coordinates of the binding residues of the holo structure (only on the holo page) |
Coordinate files are in PDB format. | | (click anywhere in this window to remove it) |
|
|
|
|
|
Table describing the holo-structures and ligands used to define
the binding sites.
Column 1 gives the PDB ID of the holo-structure.
Column 2 gives the unique ID of the ligand;
a space-separated list of HET-groups that constitute
the ligand (see Methods).
Each HET-group in the ligand is uniquely identified by
a string in which the first four characters are the three-letter
HET ID from the PDB file followed by the chain ID from
the PISA file, and the last four characters are the residue sequence
number from the PDB file.
Column 3 gives the number of atoms in each ligand.
Column 4 gives the number of protein-ligand inter-atomic
contacts. | | (click anywhere in this window to remove it) |
|
| pdb ID |
Ligand Unique ID |
#atoms |
#contacts |
| 2rmb |
ABAF___2 DMTP___1 MLEF___4 DMTF___1 MLEP___4 ABAP___2 SARF___3 SARP___3 |
68 |
196 |
Details |
|
ABAJ___2 MLEJ___4 ABAR___2 SARJ___3 SARR___3 MLER___4 DMTJ___1 DMTR___1 |
68 |
200 |
|
ABAD___2 MLET___4 SART___3 DMTT___1 MLED___4 DMTD___1 SARD___3 ABAT___2 |
68 |
197 |
|
ABAB___2 MLEL___4 SARL___3 SARB___3 DMTL___1 ABAL___2 DMTB___1 MLEB___4 |
68 |
199 |
|
ABAH___2 MLEH___4 MLEN___4 DMTN___1 ABAN___2 SARN___3 DMTH___1 SARH___3 |
68 |
194 |
| 1cwb |
ABAB___2 DMTB___1 MLEB___4 SARB___3 |
34 |
81 |
Details |
| 3odl |
ABAB___6 MVAB___4 SARB___7 YYAB___5 MLEB___2 DALB___1 MLEB___3 MLEB___8 |
65 |
150 |
Details |
| 2rma |
ABAF___2 BMTP___1 MLEF___4 MLEP___4 ABAP___2 BMTF___1 SARF___3 SARP___3 |
66 |
197 |
Details |
|
ABAH___2 BMTR___1 MLEH___4 ABAR___2 SARR___3 MLER___4 BMTH___1 SARH___3 |
66 |
199 |
|
ABAJ___2 MLEJ___4 MLET___4 BMTT___1 SART___3 SARJ___3 ABAT___2 BMTJ___1 |
66 |
189 |
|
ABAB___2 MLEL___4 SARL___3 BMTB___1 SARB___3 ABAL___2 BMTL___1 MLEB___4 |
66 |
190 |
|
ABAD___2 BMTD___1 MLEN___4 MLED___4 BMTN___1 ABAN___2 SARD___3 SARN___3 |
66 |
196 |
| 3cys |
ABAB_202 SARB_203 BMTB_201 MLEB_204 |
33 |
82 |
Details |
| 1cwl |
ABAB___2 MHLB___4 SARB___3 BMTB___1 |
34 |
80 |
Details |
| 1cwh |
ABAB___2 DSEB___3 MLEB___4 BMTB___1 |
35 |
78 |
Details |
| 1cwc |
BMTB___1 DBBB___2 SARB___3 MNLB___4 |
34 |
76 |
Details |
| 1ynd |
SFAB_401 SFAD_402 |
156 |
348 |
Details |
| 1mf8 |
ABAG___2 BMTG___1 MLEG___4 SARG___3 |
33 |
126 |
Details |
| 1mik |
AA4B___2 MLEB___4 SARB___3 BMTB___1 |
35 |
94 |
Details |
| 3odi |
ABAB___6 MVAB___4 SARB___7 MLEB___2 DALB___1 MLEB___3 XXAB___5 MLEB___8 |
65 |
158 |
Details |
| 1nmk |
SFMA_166 SFMB_201 |
106 |
286 |
Details |
| 1cwm |
ABAB___2 TBMB___1 IMLB___4 SARB___3 |
33 |
74 |
Details |
| 1cwa |
ABAB___2 MLEB___4 SARB___3 BMTB___1 |
33 |
75 |
Details |
|
|
|
|
v9.7
April 2012 |
Interdisciplinary Research Institute, Computational Biology, Villeneuve d'Ascq, France
University College London, Biomolecular Structure and Modelling Unit, London, UK
Hospital for Sick Children and University of Toronto, Structural Biology and Biochemistry Program, Toronto, Canada |
| Script execution time: 0.1777 seconds |

